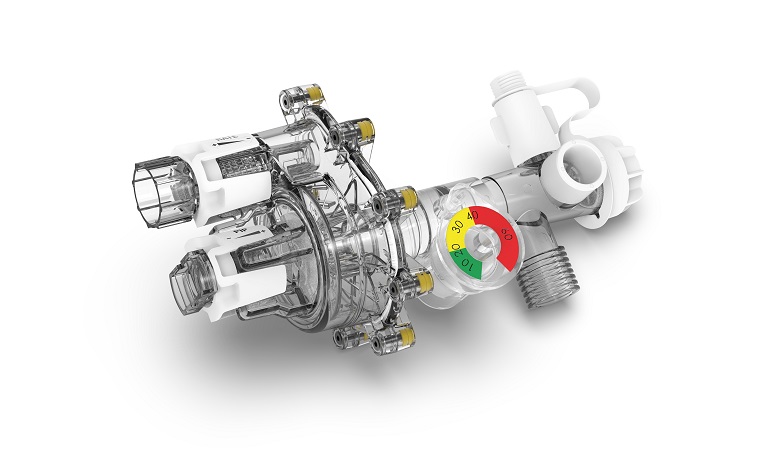
Belkin has partnered with the University of Illinois for the design of the FlexVent gas operated ventilator and Belkin’s production of the FlexVent, pending the review and approval by Food and Drug Administration (FDA).
Belkin obtained a license to the RapidVent design from the University of Illinois. Team members from both the University of Illinois and leading health care system, Carle Health, offered feedback on product design, manufacturability, training for physicians, and potential clinical scenarios where the FlexVent may be needed to help COVID-19 patients when other FDA-cleared or approved conventional/standard full-featured ventilators are unavailable.
In response to the ongoing demand for additional critical respiratory care supplies worldwide, the FlexVent will be used as a single-use, emergency ventilator that can provide constant-flow, pressure-cycled ventilation automatically to patients.
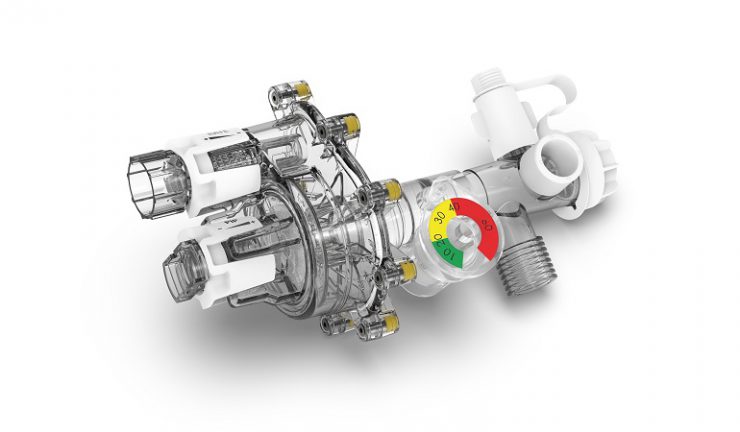
Belkin CEO and founder, Chet Pipkin said the number one responsibility is the care and compassion for others in need.
“Our merger with Foxconn Interconnect Technology (FIT) in 2018 provided us with access to the most powerful and capable manufacturing assets in the world and its long-term strategy to create new end markets in automotive, industrial and medical systems industries.
“With a global pandemic underway, we quickly realigned our assets to serve the healthcare community and were able to adapt to identify one of the most pressing needs facing them: ventilators.”